Integrated Science Questions by Topics | Introduction to integrated science, measurement, living and non-living things and matter

Integrated science questions by topics
Integrated Science Questions by Topics | Introduction to integrated science, measurement, living and non-living things and matter
Integrated science questions by topics: These are questions after each topic in the integrated science. These integrated science questions by topics can guide both teachers and learners towards any end of term examination.
Unit 1: Introduction to integrated science | Integrated science questions by topics
Part one: Objectives
Integrated Science Questions by Topics | Introduction to integrated science
1. One of the following is the reason why we do not not add water to acid but rather acid to water
a. one could slip off and cause breakages or spill solution or get burnt by solution or hurt oneself.
b. Such a practice may generate heat or explosion
c. The vapor of the mixture could be inhaled
d. The liquid may be corrosive and burn the hand
2. A scientist who researches issues relating to natural resources, plants, animals and humans is called
a. an environmental scientist b. an astronomer c. a chemist d. a medical scientist
3. What is the name of the scientist who discovered radioactivity
a. Alexander Flemming b. Dimitri Mendeleev c. Henri Becquerel d. Marie Curie
Part two: Written
Integrated Science Questions by Topics | Introduction to integrated science
4. Name two (2) prominent Ghanaian scientist
5. State five (5) careers in Science and Technology
6. Suggest four safety measures to be observed in a science laboratory
7. State the reason for observing the following laboratory rules
a. avoid smelling directly the gas evolved from chemical reaction
b. Do not open a gas tap before looking for a match to light the Bunsen burner
8. a. State five importance of Science and Technology
b. What is a scientific method
c. List the steps in scientific method
9. There is an outbreak of malaria in your community; outline the scientific method you would use to control the outbreak
Unit 2: Measurement
Part 1: Objectives
Integrated Science Questions by Topics | Measurement
1. The system of units which is accepted by all countries in the world is called
a. metric unit system b. imperial unit system c. S.I unit system d. comprehensive unit system
2. Which of the following is not a physical quantity of the basic units
a. electric current b. electric charge c. mass d. time
3. What is the equivalent of 2 g/cm3 in kg/m3
a. 2 x 10-3kg/m3 2 x 103 kgm3 c. 2 x 10-2 kgm3 d. 2 x 102 kg/m3
Part 2: Written
Integrated Science Questions by Topics | Measurement
4. Name the instruments used to measure each of the following
a. the thickness of a sheet of paper b. the diameter of a ball bearing
c. the length of a matchbox d. the diameter of a thin copper wire
5. Explain how the following S.I units are derived a. m2 b. ms-2
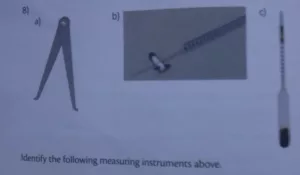
6. Identify the following measuring instruments above
Unit 3: Living and non-living things
Part 1: Objectives
Integrated Science Questions by Topics | Living and non-living things
1. Which of the following is the lowest rank of biological classification
a. kingdom b. species c. order d. genius
2. Which of the following is not a life process
a. movement b. nutrition c. flooding d. sensitivity
3. Which of the following scientists is linked to binomial nomenclature
a. Aristotle b. Charles Darwin c. Carolus Linnaues d. Gregor Mendel
Part 2: Written
Integrated Science Questions by Topics | Living and non-living things
4. a. Name the five major kingdoms in the classification of living organisms
b. Give one organism in each of the kingdoms
c. Tabulate four differences between plants and animals
d. Enumerate three similarities between plants and animals
e. State five differences between living and non-living things
5. Arrange the following social groups in decreasing order; starting from the largest group to the lowest:
extended family, ethnic group, Clan, Class and Nuclear family
6. a. Arrange the following ranks of classification of organisms in descending order; starting from the largest to the lowest: class, kingdom, phylum/division, family, genius, species and order
b. state two disadvantages of classifying elements into metal and non-metals
7. a. What is biological classification give reasons why classification is important.
b. List four (4) characteristics each for organisms in the major kingdoms
c. Give three (3) examples of organisms in each of the kingdoms named in (b) above
Unit 4: Matter
Part 1: Objectives
Integrated Science Questions by Topics | Matter
1. The arrangement in the shells of an aluminum atom is represented as
a. 2, 8, 1 b. 2, 8, 2 c. 2, 8, 3 d. 2, 8, 4
2. Nucleons are made up of
a. electrons and protons b. protons and shells c. neutrons and shells d. protons and neutrons
3. What is the molarity of 20 g of glucose per dm
a. 0.01 M b. 0.11 M c. 0.25 M d. 0.02 M
Part 2: Written
Integrated Science Questions by Topics | Matter
4. Classify each of the following as an element, a compound or a mixture. Explain your choice answer
a. Butter bread b. Distilled water c. Gold d. Air
5. With the aid of a diagram, describe how ionic bond is formed in MgO
6. The following table describes four atoms
| Atom M | Atom N | Atom O | Atom P | |
| Number of protons | 10 | 11 | 11 | 10 |
| Number of neutrons | 11 | 10 | 11 | 10 |
| Number of electrons | 10 | 11 | 11 | 10 |
a. are atoms M and N isotopes of the same element
b. are atoms M and P isotopes of the same elements
c. What is the mass number for atom M
d. What is the mass number for atom P
7. a. The mole is simply a convenient unit for counting atoms and molecules. Name four counting units that you commonly encounter in everyday life
b. Calculate the number of moles represented by each of the following
a. 16.0g of Na b. 20.0g of Ca c. 15.0g of Mg d. 0.013g of K
8. Calculate the molar mass of the following compounds
a. H2SO4
b. NaOH
c. CaCO3
d. Ca(OH)2
(H = 1.0, C = 12.0, O = 16.0, Na = 23.0, Ca = 40.0)
9. a. What is the number of atoms in 25.9 g of Al
b. Calculate the mass in grams of the following i. 0.594 mol S ii. 0.4 mol KCl
(S = 32.0, Cl = 35.5, K = 39.0)
10. a. State three household substances that can be diluted
b. Explain why sugar dissolves faster in warm water than cold water
See also: UCC JHS BECE Integrated Science Mock Questions
Post Disclaimer
The information contained in this post is for general information purposes only. The information is provided by Educative News and while we endeavour to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the post for any purpose.